SEE EARLY signs of CPAP failure in RDS, START EARLY rescue
Early rescue (<2 hours after birth) may improve outcomes in preterm infants with RDS compared with delayed rescue (>2 hours after birth)1
Early rescue (<2 hours after birth) may improve outcomes in preterm infants with RDS compared with delayed rescue (>2 hours after birth)1
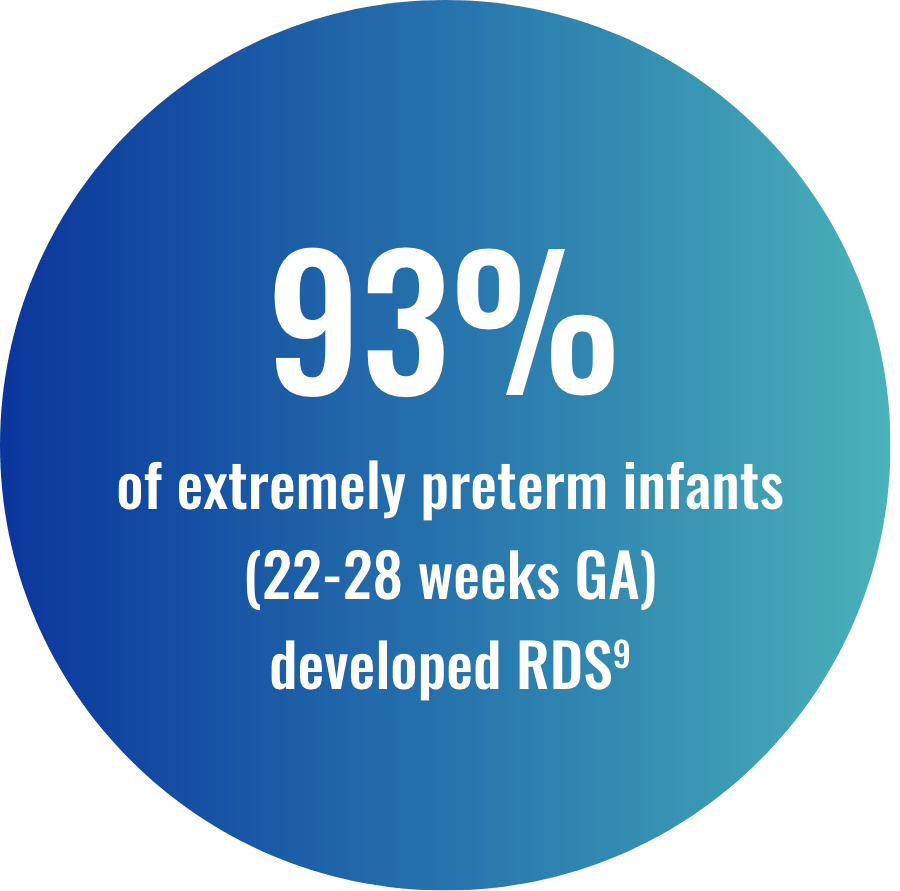
For premature infants, recognizing the earliest signs and symptoms of RDS is important. Respiratory support within the first few hours of birth may include CPAP and early rescue8,10
 ‡The European Consensus Guidelines on the
management of RDS reflect the clinical practice standards and medications approved for use in EU
hospitals; as such, certain recommendations may not be relevant to US clinical practice. US and EU
patient populations and clinical practice standards are widely accepted to be distinct; therefore, there
may be significant limitations to the extrapolation of certain data from EU studies to the US patient
population.
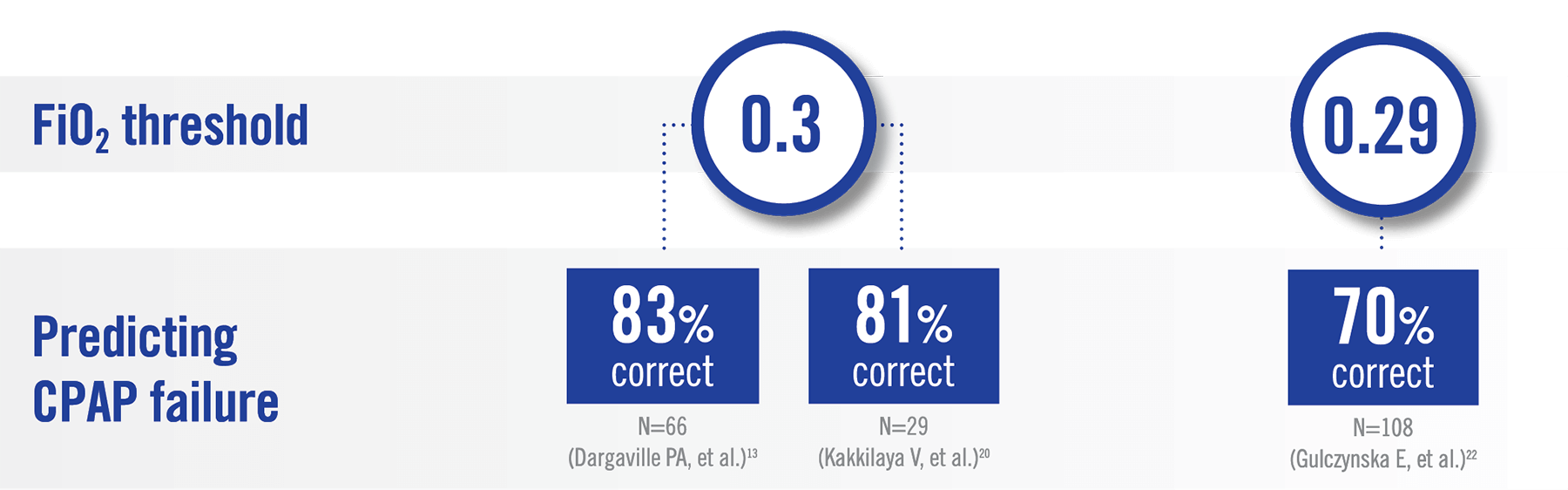
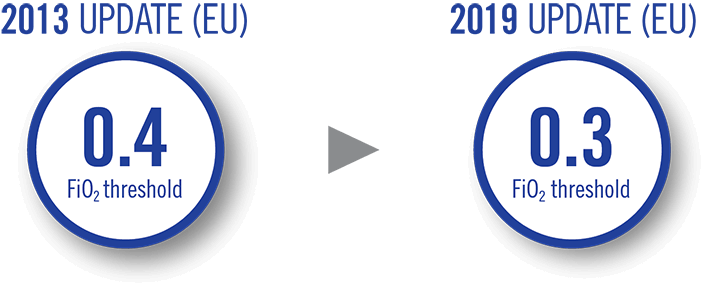
European Consensus Guidelines on RDS management have been updated to reflect earlier surfactant
administration based on lower FiO2 levels (0.3) to help optimize the timing of surfactant
treatment
‡The European Consensus Guidelines on the
management of RDS reflect the clinical practice standards and medications approved for use in EU
hospitals; as such, certain recommendations may not be relevant to US clinical practice. US and EU
patient populations and clinical practice standards are widely accepted to be distinct; therefore, there
may be significant limitations to the extrapolation of certain data from EU studies to the US patient
population.
European Consensus Guidelines on RDS management have been updated to reflect earlier surfactant
administration based on lower FiO2 levels (0.3) to help optimize the timing of surfactant
treatment
§It is important to note that the INSURE strategy may not be appropriate for all infants. Infants with RDS may vary markedly in the severity of respiratory disease, maturity, and presence of other complications, and thus it is necessary to individualize patient care.
AAP=American Association of Pediatrics; BPD=bronchopulmonary dysplasia; CPAP=continuous positive airway pressure; EU=European Union; FiO2=fraction of inspired oxygen; GA=gestational age; HOL=hours of life; NICU=neonatal intensive care unit; RDS=respiratory distress syndrome; ROC=receiver operating characteristic; SpO2=oxygen saturation.
CUROSURF® (poractant alfa) is intended for intratracheal use only. The administration of exogenous surfactants, including CUROSURF, can rapidly affect oxygenation and lung compliance. Therefore, infants receiving CUROSURF should receive frequent clinical and laboratory assessments so that oxygen and ventilatory support can be modified to respond to respiratory changes.
CUROSURF should only be administered by those trained and experienced in the care, resuscitation, and stabilization of preterm infants.
Transient adverse reactions associated with administration of CUROSURF include bradycardia, hypotension, endotracheal tube blockage, and oxygen desaturation. These events require stopping CUROSURF administration and taking appropriate measures to alleviate the condition. After the patient is stable, dosing may proceed with appropriate monitoring.
Pulmonary hemorrhage, a known complication of premature birth and very low birth-weight, has been reported with CUROSURF. The rates of common complications of prematurity observed in a multicenter single-dose study that enrolled infants 700–2000 g birth weight with RDS requiring mechanical ventilation and FiO2 ≥ 0.60 are as follows for CUROSURF 2.5 mL/kg (200 mg/kg) (n=78) and control (n=66; no surfactant) respectively: acquired pneumonia (17% vs. 21%), acquired septicemia (14% vs. 18%), bronchopulmonary dysplasia (18% vs. 22%), intracranial hemorrhage (51% vs. 64%), patent ductus arteriosus (60% vs. 48%), pneumothorax (21% vs. 36%) and pulmonary interstitial emphysema (21% vs. 38%).
CUROSURF® (poractant alfa) Intratracheal Suspension is indicated for the rescue treatment of Respiratory Distress Syndrome (RDS) in premature infants. CUROSURF reduces mortality and pneumothoraces associated with RDS.
Please see Full Prescribing Information.
References: 1. Bahadue FL, Soll R. Cochrane Database Syst Rev. 2012;11(11):CD001456. 2. Fuchs H, Lindner W, Leiprecht A, Mendler MR, Hummler HD. Arch Dis Child Fetal Neonatal Ed. 2011;96(5):F343-F347. 3. Stevens TP, Harrington EW, Blennow M, Soll RF. Cochrane Database Syst Rev. 2007;2007(4):CD003063. 4. Nanda D, Nangia S, Thukral A, Yadav CP. Eur J Pediatr. 2020;179(4):603-610. 5. Tian T, Wang L, Ye R, Liu J, Ren A. Pregnancy Hypertens. 2020;19:131-137. 6. Fathi O, Bapat R, Shepherd EG, et al. In: Chubarova AI, ed. Neonatal Medicine. 2019. Accessed September 15, 2022. https://www.intechopen.com/chapters/64996 7. Lagoski M, Hamvas A. Surfactant therapy. In: Jain L, Suresh GK. eds. Clinical Guidelines in Neonatology. McGraw-Hill Education; 2019. 8. Polin RA, Carlo WA; Committee on Fetus and Newborn; American Academy of Pediatrics. Pediatrics. 2014;133:156-163. 9. Stoll BJ, Hansen NI, Bell EF, et al. Pediatrics. 2010;126(3):443-456. 10. Committee on Fetus and Newborn, American Academy of Pediatrics. Pediatrics. 2014;133(1):171-174. 11. Annibale DJ, Bissinger RL. Adv Neonatal Care. 2010;10(5):221-222. 12. Dargaville, PA, Gerber A, Johansson S, et al. Pediatrics. 2016;138(1):e20153985. 13. Dargaville PA, Aiyappan A, De Paoli AG, et al. Neonatology. 2013;104(1):8-14. 14. Terfa ZG, Nantanda R, Lesosky M, et al. BMJ Open. 2022;12:e050729. 15. Schwartz CI, Zieve D. MedLinePlus: Medical Encyclopedia. April 14, 2021. Accessed July 26, 2022. https://medlineplus.gov/ency/article/001563.htm 16. Sweet DG, Carnielli V, Greisen G, et al. Neonatology. 2019;115(4):432-450. 17. Gyamfi- Bannerman C, Thom EA, Blackwell SC, et al. N Engl J Med. 2016;374(14):1311-1320. 18. Pillai MS, Sankar MJ, Mani K, et al. J Trop Pediatr. 2011;57(4):274-279. 19. Ovesen P, Rasmussen S, Kesmodel U. Obstet Gynecol. 2011;118(2 Pt 1):305-312. 20. Kakkilaya V, Wagner S, Mangona KLM, et al. J Perinatol. 2019;39(8):1081-1088. 21. Rojas MA, Lozano JM, Rojas MX, et al. Pediatrics. 2009;123:137-142. 22. Gulczyńska E, Szczapa T, Hozejowski R, et al. Neonatology. 2019;116(2):171-178.